IUPAC name
N-(2-chloro-6-methylphenyl)-2-({6-[4-(2-hydroxyethyl)piperazin-1-yl]-2-methylpyrimidin-4-yl}amino)-1,3-thiazole-5-carboxamide

N-(2-chloro-6-methylphenyl)-2-({6-[4-(2-hydroxyethyl)piperazin-1-yl]-2-methylpyrimidin-4-yl}amino)-1,3-thiazole-5-carboxamide
302962-49-8
488.01 g·mol−1
C22H26ClN7O2S
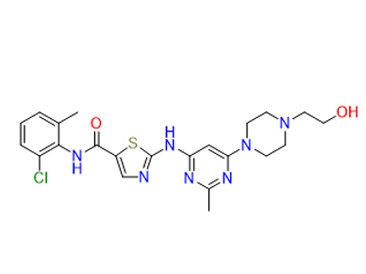
Dasatinib, at nanomolar concentrations can inhibit the following kinases: BCR-ABL, SRC family (SRC, LCK, YES, FYN), c-KIT, EPHA2, and PDGFRβ. Based on modeling studies, it is predicted to bind to multiple conformations of the ABL kinase. In vitro, it is active in leukemic cell lines representing variants of imatinib mesylate sensitive and resistant disease. Dasatinib can inhibit the growth of chronic myeloid leukemia (CML) and acute lymphoblastic leukemia (ALL) cell lines overexpressing BCR-ABL. Dasatinib is able to overcome imatinib resistance resulting from BCR-ABL kinase domain mutations, activation of alternate signaling pathways involving the SRC family kinases (LYN, HCK), and multi-drug resistance gene overexpression.