IUPAC name
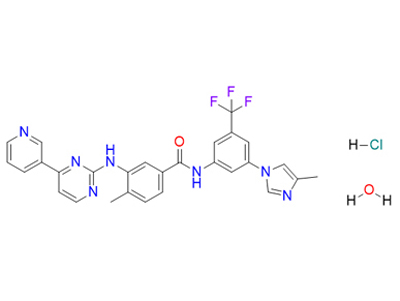
4-methyl-N-[3-(4-methylimidazol-1-yl)-5-(trifluoromethyl)phenyl]-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino]benzamide;hydrate;hydrochloride

4-methyl-N-[3-(4-methylimidazol-1-yl)-5-(trifluoromethyl)phenyl]-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino]benzamide;hydrate;hydrochloride
923288-90-8
584.0 g·mol−1
C28H25ClF3N7O2
Granule: Available
Ready to press o
Ready to fill þ