Parsian pharama review of CDK4-6 inhibitors citing to the recent articles
One of the most common cancers among women is Breast cancer. Sometimes the average risk of developing breast cancer in a woman in the United States in her lifespan is about 13%. More than 70% of breast cancer cases are Hormone receptor–positive (HR+) types, which historically have been treated with endocrine therapy, such as selective estrogen receptor inhibitors and aromatase inhibitors. Although endocrine therapy still is the backbone of HR+ breast cancer treatment, but up to 50% of patients with advanced breast cancer develop resistance. Targeted therapies like CDK4/6 inhibitors have been approved to combat resistance in HR+ breast cancer patients.
CDK4/6 inhibitors, the newest class of interest for advanced breast cancer
The newest class of interest for advanced breast cancer, Cyclin-dependent kinase inhibitors, work by specifically inhibiting CDK4/6 proteins and blocking the transition from G1-to-S phase which is crucial for normal and cancer cell proliferation. The developing and approval of Cyclin Dependent Kinase 4 and 6 inhibitors (CDK 4/6) has substantially changed therapeutic approach for metastatic hormone receptor (HR) positive breast cancer.
The addition of these agents to standard endocrine therapy has been evaluated in Phase II–III clinical trials and shows consistent improvements in response rates, excellent impact on patients’ quality of life and progression-free survival as well as manageable toxicity profiles.
Based on the results obtained in pivotal trials, three CDK4/6 inhibitors namely; palbocilib, ribociclib, and abemaciclib are United Stated Food and Drug Administration (FDA) and European Medicines Agency (EMA)approved for HR+, (HER2–), advanced or metastatic breast cancers in combination with aromatase inhibitors or fulvestrant both in first, second, and beyond lines of therapy.
Choosing a CDK4/6 inhibitor: Which one is a better treatment option for you?
So far, none of CDK-Is, such as Palbociclib, ribociclib and abemaciclib have been directly compared to each other in a clinical trial, so they are considered to work equally well. The treatment team can discuss about the most proper treatment depending on each and every one’s situation.
Fundamental criteria have to be taken into account for considering the best treatment option. Such as:
- the stage of the BC
- any treatments that you have had in the past
- How fast the cancer has grown after previous treatment
- if you are a woman, whether you are postmenopausal or premenopausal
- the adverse effect associated with each CDK4/6 inhibitor
- One’s treatment team preference that the patient have to take medication continuously, or on a 3-week on/ 1-week off cycle
- A patient’s insurance monetary support toward specific medicine
Every one of the CDK4/6-Is are approved by FDA to treat certain stages of HR+ HER2-negative breast cancer in different groups of patients.
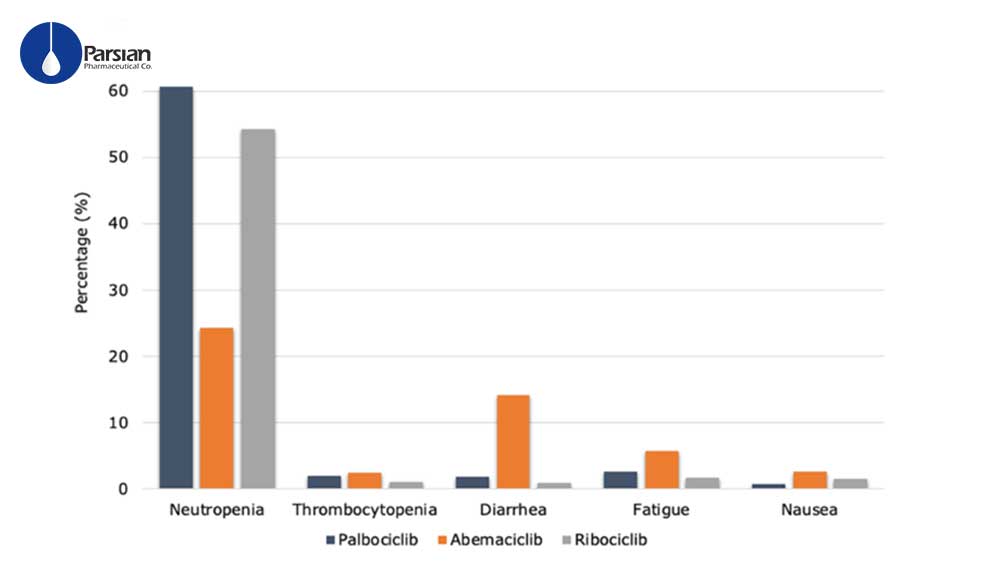
CDK4/6-Is Like the other cancer medicines cause adverse effects, but less intense than those caused by chemotherapy. The most common adverse effects of all three medicines are:
diarrhea, nausea, fatigue, anemia (low red blood cell counts),low platelet counts, low white blood cell counts (also called neutropenia).
Due to patient’s situation, physicians have to observe that certain adverse effects tend to be more pronounced with some CDK4/6-Is than others and this might influence treatment decisions.
For example, abemaciclib is associated with a significant risk of gastrointestinal problems. For instance, diarrhea is common, and in about 10% of patients can be severe and might lead to dehydration or infection. In some cases, your physicians may lower your dose of abemaciclib or recommend that you take a break from treatment. If you already have GI problems such as; IBS, colitis, diverticulitis, or frequent diarrhea, among others your physician may recommend one of the other CDK4/6-Is.
In rare cases, ribociclib can cause QT interval prolongation. This can lead to a life-threatening irregular or fast heartbeat. Patients who are on ribociclib during the first few cycles of treatment, need to have an EKG every couple of weeks. If you already have a heart problem, your treatment team may recommend one of the other CDK4/6-Is.
Switching between CDK4/6 inhibitors
Although none of the CDK4/6-Is have been directly compared to each other in a clinical trial, physicias consider them to work equally well. So if you experience problematic side effects with one of the CDK4/6-Is, even after the dose is adjusted, you and your treatment team can think about switching to a different one.
No study results suggest that another CDK4/6 inhibitor will be effective, if the cancer gets worse while you are on a CDK4/6 inhibitor. Your physician may change the hormonal therapy you are taking in combination with. In certain cases, your physician may decide whether it is worth trying a different CDK4/6 inhibitor or not.
Also Parsian pharma has some tips for Patients who are taking a CDK4/6 inhibitor:
You can find out more treatment options for Breast cancer in recent Parsian pharma article: aromatase inhibitors
Some of the main reference we have observe them: